Past Issues
Spinal Dural Arteriovenous Fistulas: Surgical Outcome Analysis of Twenty Cases
MAA Salek1,*, MS Rana,2 MM Hasan,2 AH Manik,1 MS Islam2
1Department of Neurosurgery, Combined Military Hospital Dhaka
2Department of Neurosurgery, Dhaka Medical Hospital
*Corresponding Author: Md Al Amin Salek, MBBS, MCPS, FCPS, MRCS, Combined Military Hospital Dhaka, Dhaka; Email: [email protected]
Received Date: January 17, 2023
Publication Date: March 23, 2023
Citation: Salek MAA, et al. (2023). Spinal Dural Arteriovenous Fistulas: Surgical Outcome Analysis of Twenty Cases. Neuro Research. 5(1):16.
Copyright: Salek MAA, et al. © (2023).
ABSTRACT
Background: Spinal dural arteriovenous fistulas (SDAVFs) are rare complex spinal vascular shunts, which can inevitably lead to severe disability if remain untreated. Methods: Retrospective analysis of SDAVF patients in a period of three years (2019 to 2021) who presented with progressive paraparesis, bladder symptoms, and/or sensory disturbances. They were evaluated by MRI and localization of the fistula was done by spinal catheter angiogram. Spinal AV fistulas were simply classified as extradural and intradural. They all underwent microsurgical shunt interruption. Neurological function was evaluated by Aminoff-Logue Scale (ALS). Individual ALS scores of each patient (pretreatment and post-treatment) were recorded to find out the p-value of the surgical intervention. Results: A total of 20 patients (mean age 35 ± 10 years, 16 (80.0%) are male. The mean interval from onset to diagnosis was 6 ± 3 months. Among the anatomical location of fistulas, 1(05.0%) was cervical, 16 (80.0%) were in thoracic, and 3 (15.0%) were lumbar. Among the angiographic types of fistulas, 18(90.0%) were intradural and, 2 (10.0%) were extradural. Compared with pre-operative ALS scores,15 (75.00%) patients received improvement, and 05 (25.00%) patients felt worse or more stable. Among 20 surgical procedures, there were complications in three cases (15%): epidural hematoma in 1 case, cerebrospinal fluid leakage in 1 case, and postoperative wound infection in 1 case. Conclusions: In our study, microsurgical interruption of timely diagnosed SDAVF showed good and stable results over time.
Keywords: Spinal dural arteriovenous fistula, spinal catheter angiogram
INTRODUCTION
Spinal dural arteriovenous fistula (SDAVF) is a rare disease, whose etiology is not entirely clear. It is the most common vascular malformation of the spinal cord, comprising 60-80 % of the cases [1]. They are defined as direct arteriovenous shunts in the spinal dura mater between a segmental root artery and a peri-medullary vein [2]. The etiology of SDAVFs is unknown but they are presumed to be an acquired pathology mainly affecting middle-aged men. High venous pressure leading to chronic hypoxia is presumed to be the cause of clinical symptoms [3-6]. SDAVFs are commonly located at the thoracic and lumbar levels and are responsible for progressive myelopathies with progressive sensory and motor deficits of the lower limbs associated with sphincter disturbances [7]. There is a male predominance with a sex ratio of almost 5:1 [8]. SDAVFs are considered a curable cause of myelopathy. SDAVF treatment consists of interrupting the shunt between the artery and the vein either surgically or endovascularly. The main surgical difficulty is locating the origin of the shunting vein [9].
The main objective of this study was to describe the clinical outcome of SDAF patients who were treated surgically. The secondary objectives were to find out the failure rate, late recurrence rate, and complication rate of surgery.
MATERIALS AND METHODS
We conducted a retrospective observational study. The medical records of 20 consecutive patients with SDAVFs treated in 2 academic neurosurgical departments (Combined Military Hospital Dhaka and Dhaka Medical College Hospital) between 2019 and 2021. Inclusion criteria were patients treated for SDAVFs surgically. Exclusion criteria were SDAVFs that were treated endovascularly and those that were not treated.
Clinical data: Age at diagnosis, gender, and duration of symptoms were collected for each patient. The onset of symptoms was when neurological deficits (gait disturbances, bladder incontinence) were first noticed. The functional status of the patients was assessed using the Aminoff and Logue’s Scale (ALS, which grades gait and urinary incontinence before and after the surgery.
Radiological data: The level of intramedullary hyperintensity and flow voids on T2-weighted MRI was measured using the corresponding number of vertebral bodies. Fistula level was identified by selective catheter spinal arteriography.
Treatment: All selected patients underwent surgical closure of the fistula. The initial success of treatment, recurrence, and complications was reviewed. The surgical procedure consisted of a laminectomy centered on the arteriovenous shunt under the guidance of an image intensifier, the opening of the dura, and the exclusion of the fistula at the origin of the draining vein. Recurrence was defined as a symptomatic re-opening of the fistula after an initial successful exclusion.
Primary endpoint: The primary objective of the study was to observe the neurological outcome between the pre-treatment ALS and the last examination of ALS.
Secondary endpoints: Secondary endpoints were to determine the recurrence and complication rates.
Statistical analysis: Descriptive statistics were used for age, gender, anatomical location and angiographic typing, pre-treatment, and last examination of ALS. Linear mixed models were used for the primary objective. Comparisons between pre-treatment and last examination ALS for each subgroup were made using the Tukey Test. The significance threshold was set at P ≤ 0.05.
Ethical approval: This study was reviewed and approved by the ethics committee of our institution.
RESULTS
Patient characteristics and treatment method: A total of 20 surgical procedures were performed: 18 as an initial treatment, 1 after an initial failed embolization attempt, and 1 after the failed initial surgery. (Figure 1)
Figure 1: Flow chart of patient characteristics and treatment method.
Anatomical and angiographic findings: Among the anatomical location of fistulas, 1(05.0%) was in the cervical, 16 (80.0%) were in the thoracic, and 3 (15.0%) were in the lumbar region. Among the angiographic types of fistulas, 18(90.0%) were intradural and, 2 (10.0%) were extradural. (Figure 2)
Figure 2: Anatomical and angiographic findings.
Clinical outcome: Mean pre-treatment ALS was 4.55 and the mean Post-treatment ALS was 2.85. There was a significant improvement in ALS between pretreatment and post treatment ALS (P = 0.0009). Further analyses were made for the G score and the M score. The mean pre-treatment and post-treatment G scores were respectively 3.10 and 2.00. The G score improved significantly after surgery (P < 0.0001). Mean pre-treatment and post-treatment M scores were respectively 1.45 and 0.95 after surgery. M score did not improve significantly after the treatment. (Table 1)
Table 1: Clinical outcome after surgery.
|
Variables |
Pretreatment score |
Post-treatment score |
P value |
|
ALS |
4.55 |
2.85 |
P=0.0009 |
|
G score |
3.10 |
2.00 |
P<0.0001 |
|
M score |
1.45 |
0.95 |
Not significant |
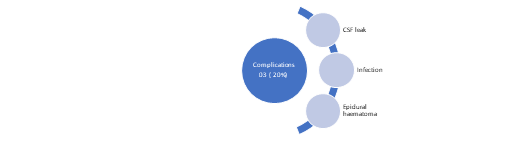
Complications: Among 20 surgical procedures there were complications in three cases (15%): epidural hematoma in 1 case which needed re-exploration, cerebrospinal fluid leakage in 1 case that s closed spontaneously, and postoperative wound infection in 1 case. (Figure 3).
Figure 3: Complications following surgery.
DISCUSSION
SDAVFs are rare and still underdiagnosed entities. They are the most frequent vascular malformation of the spine and account for approximately 70% of all vascular spinal malformations. They need proper treatment to prevent morbidity with progressive spinal cord symptoms [10].
Table 2: Aminoff and Logue scale [11].
|
Gait (G) |
Micturition (M) |
|
G0 Normal |
M0 Normal |
|
G1 Leg weakness, abnormal gait or stance, but no restriction of activity |
M1 Hesitancy, frequency, urgency |
|
G2 Restricted activity |
M2 Occasional urinary incontinence or retention |
|
G3 Requiring one stick for walking |
M3 Total incontinence or persistent retention |
|
G4 Requires two sticks, crutches, or walker G5 Confined to a wheelchair G Score = G+M score= |
M score =
|
Aminoff-Logue Scale (ALS) is used as a tool for neurological outcomes (Table 2).
Table 3: Baseline demographics with ALS.
|
Serial no |
Age |
Sex (M-16; F-04) |
ALS |
Remarks |
|
|
Pretreatment (G+M) |
Post-treatment (G+M) |
||||
|
1 |
45 |
M |
5 (3+2) |
3(2+1) |
|
|
2 |
36 |
M |
5(4+1) |
3(2+1) |
|
|
3 |
52 |
M |
4 (3+1) |
2(2+0) |
|
|
4 |
40 |
F |
3(2+1) |
3(2+1) |
Stable |
|
5 |
28 |
F |
5(3+2) |
2(2+0) |
|
|
6 |
35 |
M |
5(3+2) |
3(2+1) |
|
|
7 |
43 |
M |
2(2+0) |
2(2+0) |
Stable |
|
8 |
39 |
M |
6(4+2) |
4(3+1) |
|
|
9 |
54 |
M |
7(4+3) |
5(3+2) |
|
|
10 |
19 |
F |
4(3+1) |
2(1+1) |
|
|
11 |
38 |
M |
4(3+1) |
2(1+1) |
|
|
12 |
45 |
M |
5(3+2) |
3(2+1) |
|
|
13 |
49 |
M |
4(3+1) |
2(1+1) |
|
|
14 |
33 |
M |
3(2+1) |
3(2+1) |
Stable |
|
15 |
40 |
F |
5(3+2) |
6(4+2) |
worsen |
|
16 |
32 |
M |
6(4+2) |
4(3+1) |
|
|
17 |
44 |
M |
4(3+!) |
4(3+1) |
Stable |
|
18 |
42 |
M |
3(3+0) |
1(1+0) |
|
|
19 |
39 |
M |
5(3+2) |
2(1+1) |
|
|
20 |
48 |
M |
6(4+2) |
3(2+1) |
|
In the literature, there is a male predominance with a sex ratio of almost 5:1 [12] In our study it was 4:1. There was a significant improvement in the ALS between pretreatment and post-treatment ALS (Table-3). These results suggest that microsurgical interruption of SDAVFs is an efficient treatment option for achieving permanent occlusion using a single procedure. Lee, H.S. et al. [13] proposed surgical treatment is still the first option when endovascular therapy (EVT) is not feasible after diagnostic spinal angiography. In our setup dedicated endovascular intervention is yet to develop. In this study, the overall complication rate was 15% which was higher than that of other studies [14]. All the complication cases were managed successfully after surgery. Therefore, the complication rate should not present an obstacle to the choice of surgery. Surgical technique has improved in recent years, which has raised the occlusion rate and decreased the risk of complications. Operating microscopes have been perfected with fluorescence modules. The use of indocyanine green or fluorescein has been proven to be useful in vascular neurosurgery [15-17]. They can be used in the same way for confirmation of the angioarchitecture of the SDAVFs and to verify its occlusion at the end of the procedure [18]. Furthermore, some authors have reported that pre-operative identification of the level of the SDAVF can be carried out by placing a coil in the feeding artery during the pre-operative spinal arteriography. This technique appears to decrease the risk of error at the vertebral level [19]. Finally, a mini-invasive technique with limited surgical exposure could reduce the risk of infection and decrease the length of hospital stay [20]. Our study has some limitations. The patient sample was small due to the rarity of this pathology. Our clinical endpoint (ALS) was calculated retrospectively which was a potential bias.
CONCLUSION
Surgery may be considered as a first-line treatment in SDAVFs considering the straightforward technical aspects and favorable clinicopathological outcome.
REFERENCES
- Maimon S, Luckman Y, Strauss I. (2016). Spinal Dural Arteriovenous Fistula: A Review. Adv Tech Stand Neurosurg. 43:111-137.
- Kim LJ, Spetzler RF. (2006). Classification and Surgical Management of Spinal Arteriovenous Lesions: Arteriovenous Fistulae and Arteriovenous Malformations. Neurosurgery. 59:195-201.
- Rosenblum B, Oldfield EH, Doppman JL, Di Chiro G. (1987). Spinal arteriovenous malformations: a comparison of dural arteriovenous fistulas and intradural AVMs in 81 patients. J Neurosurg. 67:795-802.
- Aminoff MJ, Barnard RO, Logue V. (1974). The pathophysiology of spinal vascular malformations. J Neurol Sci. 23:255-263.
- Kendall BE, Logue V. (1977). Spinal epidural angiomatous malformations draining into intrathecal veins. Neuroradiology. 13:181-189.
- Hassler W, Thron A, Grote EH. (1989). Hemodynamics of spinal dural arteriovenous fistulas. An intraoperative study Neurosurg. 70:360-370.
- Cenzato M, Debernardi A, Stefani R, D’Aliberti G, Piparo M, Talamonti G, et al. (2012). Spinal dural arteriovenous fistulas: outcome and prognostic factors. Neurosurg Focus. 32:E11.
- Ropper AE, Gross BA, Du R. (2012). Surgical treatment of Type I spinal dural arteriovenous fistulas. Neurosurg Focus. 32:E3.
- Kirsch M, Berg-Dammer E, Musahl C, Bäzner H, Kühne D, Henkes H. (2013). Endovascular management of spinal dural arteriovenous fistulas in 78 patients. Neuroradiology. 55:337-343.
- Krings T, Geibprasert S. (2009). Spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol. 30(4):639-648.
- Aminoff MJ, Logue V. (1974). Clinical features of spinal vascular malformations. Brain. 97:197-210.
- Nagata S, Morioka T, Natori Y, Matsukado K, Sasaki T, Yamada T. (2006). Factors that affect the surgical outcomes of spinal dural arteriovenous fistulas. Surg Neurol. 65:563-568.
- Lee HS, Kang HS, Kim SM, Kim CH, Yang SH, Han MH, et al. (2021). Treatment strategy to maximize the treatment outcome of spinal dural arteriovenous fistula after initial endovascular embolization attempt at diagnostic angiography. Sci Rep 11:10004.
- Muralidharan R, Mandrekar J, Lanzino G, Atkinson JL, Rabinstein AA. (2013). Prognostic value of clinical and radiological signs in the postoperative outcome of spinal dural arteriovenous fistula. Spine. 14:1188-1193.
- Bretonnier M, Henaux PL, Morandi X, Le Reste PJ. (2018). Fluorescein-guided resection of brain arteriovenous malformations: A short series. J Clin Neurosci. 52:37-40.
- Schuette AJ, Cawley CM, Barrow DL. (2010). Indocyanine green video angiography in the management of dural arteriovenous fistulae. Neurosurgery. 67:658-662.
- Spiotta AM, Bain M, Moskowitz S. (2011). Intraoperative indocyanine green angiography as a substitute for conventional angiography in the surgical management of spinal dural arteriovenous fistulae. J Neurointerv Surg. 3:182-185.
- Zaidi HA, Abla AA, Nakaji P, Chowdhry SA, Albuquerque FC, Spetzler RF. (2014). Indocyanine green angiography in the surgical management of cerebral arteriovenous malformations: lessons learned in 130 consecutive cases. Neurosurgery. 10:246-251.
- Marquardt G, Berkefeld J, Seifert V, Gerlach R. (2009). Preoperative coil marking to facilitate intraoperative localization of spinal dural arteriovenous fistulas. Eur Spine J. 18:1117-1120.
- Patel NP, Birch BD, Lyons MK, DeMent SE, Elbert GA. (2013). Minimally invasive intradural spinal dural arteriovenous fistula ligation. World Neurosurg. 80:267-270.
 Abstract
Abstract  PDF
PDF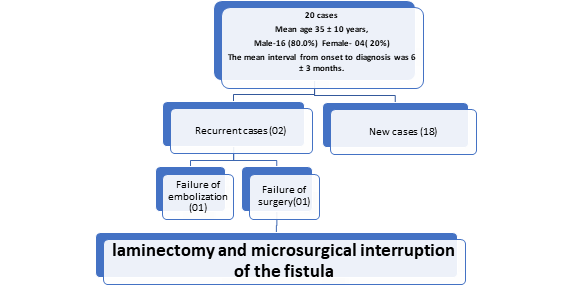
.png)