Past Issues
The Diagnostic Value of Mattis Dementia Rating Scale- Initiation Perseveration Subscale in Vascular Cognitive Disorders
George P Paraskevas1*, Elisabeth Kapaki1, Vasilios C Constantinides1, George Liakakis1, Ilia Theotoka1, Panagiotis G Paraskevas2, Ioannis Zalonis1
1Department of Medicine, National and Kapodistrian University of Athens, Greece 2Department of Nursing, School of Health and Welfare Services, Technological Educational Institute of Crete, Greece
*Corresponding Author: George P Paraskevas, Division of Cognitive and Movement Disorders and Unit of Neurochemistry and Biological Markers, 1st Department of Neurology, National and Kapodistrian University of Athens, School of Medicine, Eginition Hospital, 72 Vas. Sophias Ave, Athens 11528, Greece.
Received: Aug 16, 2019 Published: Sep 6, 2019
ABSTRACT
Vascular cognitive disorder (VCD)2 comprises a heterogeneous group of cerebrovascular disorders, leading to a continuum of cognitive impairment, ranging from the level of mild cognitive impairment or mild vascular cognitive disorder (VCDM) to full-blown vascular dementia or major vascular cognitive disorder (VCDD). The Mattis Dementia Rating Scale-Initiation Perseveration subscale (MDRS-IP) may be useful for the assessment of frontal dysfunction, which is significantly affected in most patients with VCD. The aim of the present study was to explore the diagnostic value of MDRS-IP in patients with VCD of various severity. In a total of 140 patients fulfilling most recent criteria for VCDD and VCDM or having cerebrovascular disease with no clinically obvious cognitive decline (vascular controls, VC) and 15 healthy controls (Ctrl) we administered the Mini Mental State Examination (MMSE) and the MDRS-IP. Both MMSE and MDRS-IP perform better at the dementia level of VCD (VCDD), being effective in the discrimination of vascular dementia from the Ctrl or VC groups. However, in patients with VCDM, MMSE and MDRS-IP have statistically significant, but clinically moderate ability to differentiate from Ctrl or VC, i.e., to identify cognitive dysfunction in early, pre-dementia stages. Finally, both tests failed in the discrimination between VC and Ctrl, indicating that they are not sensitive enough to identify patients with cerebrovascular disease and subclinical cognitive dysfunction.
Keywords: Vascular cognitive disorder, Vascular cognitive impairment, Vascular dementia, Mattis dementia rating scale, Initiation and perseveration subscale, Neuropsychological testing
INTRODUCTION
Vascular cognitive impairment [1] or vascular cognitive disorder (VCD) [2] comprises a heterogeneous group of cerebrovascular disorders, leading to a continuum of cognitive impairment, ranging from the level of mild cognitive impairment or mild vascular cognitive disorder (VCDM) to full-blown vascular dementia or major vascular cognitive disorder (VCDD) [3]. Although dysfunction of multiple cognitive domains is increasingly recognized in vascular dementia (VD), [4] requiring extensive neuropsychological testing [1,2] the frontal dysexecutive syndrome seems to be a key presenting and diagnostic feature, [5] especially in patients suffering of subcortical small vessel disease [6]. The Mattis Dementia Rating Scale (MDRS) is a commonly used global cognitive tool composed of 5 subscales, testing attention, initiation and perseveration, construction, conceptualization and memory [7]. In particular, the initiation-perseveration subscale (MDRS-IP) may be useful for the assessment of frontal executive function [8] and can be used as a short stand-alone instrument [9]. The aim of the present study was to explore the diagnostic value of MDRS-IP in patients with VCD of various severity.
MATERIALS AND METHODS
Patients
Patients were recruited according to brain Magnetic Resonance Imaging (Magnetic Philips Medical Systems-Achieva 3.0 T (TX), Amsterdam, the Netherlands, including T1, T2, fluid attenuation inversion recovery and diffusion-weighted sequences), revealing multiple (sometimes confluent) lacunar lesions in white matter and/or the basal ganglia or Biswanger’s disease, and/or multiple large vessel infarcts, due to various combinations of risk factors (hypertension, diabetes, dyslipidemia, coronary heart disease or atrial fibrillation). They all fulfilled the criteria of “significant neuroimaging evidence of cerebrovascular disease” as suggested by the International Society for Vascular Behavioural and Cognitive Disorders (VASCOG) [2]. Exclusion criteria were: (1) recent infarct on MRI or symptoms suggestive of stroke within the last 6 months or having medial temporal lobe atrophy score >2 according to a visual scale [10], (2) presence of obvious aphasia, apraxia or paresis interfering with performance to neuropsychological tests, (3) presence of confounding factors, including thyroid dysfunction, B12 or folate insufficiency, alcohol abuse or psychiatric disorder and (4) cerebrospinal fluid biomarker profile suggestive of Alzheimer’s disease coexistence (low Aβ42 and increased phospho-tau protein) [2], or at least low Aβ42, compatible with “Alzheimer’s pathological change” [11].
Finally, a total of 140 patients were enrolled in the study. Thirty eight of them (27.14%) had multiple large vessel infarcts, 87 (62.14%) had subcortical small vessel disease and 15 (10.71%) had combination of the above. They were divided in 3 groups: (a) The major VCD or vascular dementia group (VCDD) comprised 42 patients fulfilling the VASCOG criteria for probable major VCD [2]. (b) The mild VCD or vascular mild cognitive impairment group (VCDm) comprised 47 patients fulfilling the VASCOG criteria of probable mild VCD [2]. (c) The vascular control group (VC) comprised 51 patients with significant imaging evidence of cerebrovascular disease, sufficient for at least mild VCD according to the VASCOG criteria, [2] but no cognitive complaints (by the patient or knowledgeable informant) and no clinical impression of cognitive dysfunction during interview.
For comparison, a control (Ctrl) group was used comprising 15 healthy subjects without lesions in MRI and with no cardiovascular risk factors.
All subjects or legal guardians gave their written informed consent for inclusion in the study, which was performed according to the 1975 Declaration of Helsinki, revised in 2013 and had the approval of the Scientific and Ethics Committee of Eginition Hospital.
Instruments
Following routine history taking and physical (including neurological) examination, patients (and their caregivers) and controls were first approached by a semi-structured interview assessing global cognitive status, activities of daily living and behavioral symptoms. Next, they were given the MiniMental State Examination (MMSE), [12] followed by the MDRS-IP [7]. Both tests lasted no more than 20 min in all patients. In brief, the MDRS-IP has 11 items (E-O) testing verbal fluency and programming, motor programming and drawing [7]. Items do not contribute equally to the total score (maximum 37), which is heavily dependent to the score of item e testing complex verbal fluency. The latter alone, with a maximum of 20 points, accounts for 54% of the maximum total score.
Statistical analysis
Neuropsychological test scores were compared among groups by 2-way analysis of covariance (ANCOVA) with diagnostic group and sex as cofactors and age and education as covariates. This was deemed necessary in order to control for the above parameters. However, since some of the variables did not follow the normal distribution and/or variances were heterogeneous, comparisons were repeated by non parametric tests (Kruskal-Wallis test followed by individual Mann-Whitney U tests with Bonferroni correction for multiple comparisons). The Rank order Spearman correlation coefficient (RS) and multiple regression models were also used for exploring the possible effects of variables such as education, gender and age on neuropsychological test scores and for assessing concurrent validity. Internal consistency of MDRS-IP was measured by Cronbach’s α. The discriminative value of MMSE and MDRS-IP was tested by Receiver Operating Characteristics (ROC) curve analysis, followed by comparison of the areas under the curves (AUC). The level of statistical significance was set at 0.05.
RESULTS
Comparison among groups
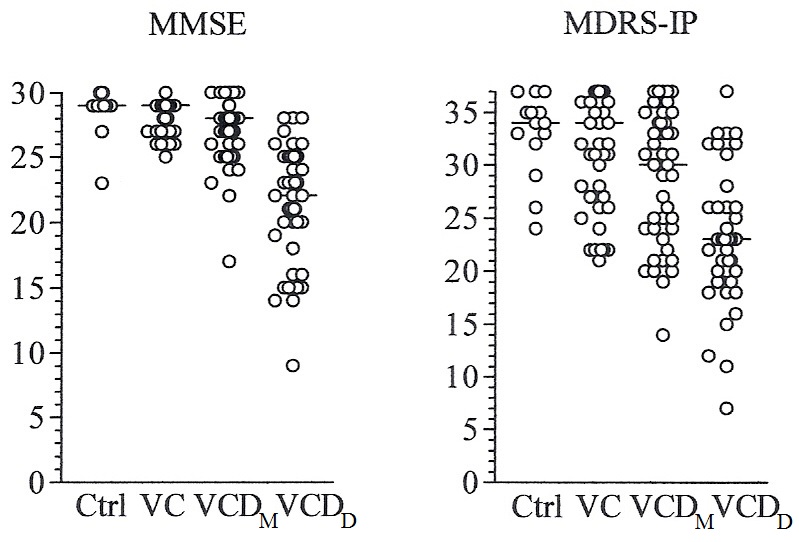
Demographic, and neuropsychological data of the studied groups are summarized in Table 1 and Figure 1. No significant difference in sex was observed. The VCDM and VCDD groups were relatively older and controls had more years of education as compared to the other groups; however, none of these numerical differences reached statistical significance. Significant differences in MMSE and MDRS-IP were observed among groups in both Kruskal-Wallis test and ANCOVA (Table 1). The MMSE score was lower in VCDM as compared to VC and lower in VCDD as compared to all the other groups; however no difference was observed between Ctrl and VC. The MMSE was affected negatively by age (P=0.016) and positively by education (P=0.012). Sex also affected MMSE scores with males performing better than females (P=0.023).
Figure 1: Scatterplot of neuropsychological test scores in the studied groups. Horizontal bars indicate median values.
The MDRS-IP score was lower in the VCDM group as compared to VC and Ctrl, and lower in VCDD as compared to all the other groups; however no difference was present between Ctrl and VC (Table 1). Minor effects of sex (but not sex by group) and education did not reach statistical significance (both P=0.09).
Table 1: Demographic characteristics and neuropsychological findings in the studied groups.
|
|
|||||
|
|
Ctrl |
VC |
VCDM |
VCDD |
P |
|
n (m/f) |
15 (8/7) |
51 (25/26) |
47 (25/22) |
42 (26/16) |
NS † |
|
Age (y) |
62.2 ± 15.0 |
63.1 ± 16.6 |
67.4 ± 12.1 |
68.0 ± 10.7 |
NS ‡ |
|
Education (y) |
14.9 ± 4.7 |
11.4 ± 4.6 |
12.1 ± 5.2 |
11.9 ± 4.3 |
NS ‡ |
|
MMSE |
28.8 ± 1.9 |
28.7 ± 1.4 |
27.2 ± 2.5 a |
21.3 ± 4.6 b |
< 0.000001 # |
|
29 (29-30) |
29 (28-30) |
28 (26-29) c |
22 (19-25) d |
< 0.0001 § |
|
|
MDRS-IP |
32.8 ± 4.3 |
31.8 ± 5.5 e |
28.8 ± 6.3 f |
23.1 ± 6.6 g |
< 0.000001 # |
|
34 (31-36) |
34 (28-37) |
28 (26-29) h |
22 (19-25) i |
< 0.0001 § |
|
|
Data are presented as mean values ± SD or as median values (25th-75th percentile). † χ2-test. ‡ One-way ANOVA. # Two-way ANCOVA followed by Newman-Keuls post-hoc tests. § Kruskal-Wallis test followed by Bonferroni-corrected Mann-Whitney U tests. a P = 0.011 vs VC. b P < 0.0001 vs Ctrl, VC and VCDM. c P = 0.002 vs VC. d P < 0.00001 vs Ctrl, VC and VCDM. e P = 0.014 vs VCDM. f P = 0.013 vs Ctrl. g P < 0.0001 vs Ctrl, VC and VCDM. h P = 0.078 vs VC. i P < 0.0001 vs Ctrl and VC and P = 0.0018 vs VCDM |
|||||
As regards MDRS-IP items, semantic verbal fluency and simple fluency were lower in VCDD as compared to all other groups (Table 2). Significant differences were also observed in the other items except for finger taping, and the second and third drawing items (Table 2).
Table 2: MDRS-IP scores in the studied groups.
|
|
Ctrl |
VC |
VCI |
VD |
P values |
|
e. Complex fluency |
16 (13-20) |
17 (11-20) |
14 (8.5-17.5) |
10 (7-13)a |
< 0.0001§ |
|
f. Simple fluency |
8 (8-8) |
8 (8-8) |
8 (6-8) |
6 (4-7)b |
< 0.0001§ |
|
g. Consonant perseveration |
1 |
1 |
0.91 |
0.77 |
0.0021† 0.0003‡ |
|
h. Vowel perseveration |
1 |
1 |
0.95 |
0.92 |
NS† 0.05‡ |
|
i. Alternating movements 1 |
1 |
0.93 |
0.88 |
0.58 |
< 0.0001† < 0.0001‡ |
|
j. Alternating movements 2 |
1 |
0.98 |
0.98 |
0.68 |
< 0.0001† < 0.0001‡ |
|
k. Finger tapping |
1 |
1 |
1 |
1 |
NS † ‡ |
|
l. Drawing 1 |
1 |
0.93 |
0.86 |
0.74 |
0.036† 0.004‡ |
|
m. Drawing 2 |
1 |
1 |
1 |
0.95 |
NS † ‡ |
|
n. Drawing 3 |
1 |
1 |
0.98 |
0.97 |
NS † ‡ |
|
o. Drawing 4 |
1 |
1 |
1 |
0.85 |
0.001† 0.0013‡ |
|
Data are presented as mean values ± SD, as median values (25th-75th percentile) or as percentage of subjects with score 1. † χ2-test. ‡ χ2-test for trend. # Two-way ANCOVA followed by Newman-Keuls post-hoc tests. § Kruskal-Wallis test followed by Bonferroni-corrected Mann-Whitney U tests. a P=0.005 vs Ctrl, 0.016 vs VCDM and < 0.0001 vs VC. b P=0.0055 vs Ctrl, 0.0013 vs VCDM and < 0.0001 vs VC |
|||||
Concurrent validity and internal consistency
The non-parametric (Spearman) rank correlation coefficient between MMSE and MDRS-IP was 0.69 (P < 0.000001). A forward stepwise multiple regression model of MDRS-IP (dependent variable) with MMSE, age, sex and education introduced as independent variables, resulted in multiple R=0.68 and adjusted R2=0.45 (P < 0.000001). The beta value of MMSE was 0.66 (P < 0.000001), with MDRS-IP being affected by sex (beta=0.16, P=0.026) and education (beta=0.14, P=0.0087).
Cronbach’s α for MDRS-IP was 0.45 with no significant improvement after deleting any of the items.
Discriminative value
Analysis of ROC curves and pairwise comparison of AUCs (Table 3) revealed that both tests offered very good discrimination between VCDD and Ctrl. For the discrimination between VCDD and VC, both MMSE and MDRS-IP performed well, with MMSE performing significantly better than MDRS-IP. Additionally, MMSE was significantly better than MDRS-IP in the discrimination between VCDD and VCDM. However, the ability of MMSE and MDRS-IP to differentiate between VCDM and Ctrl or VC was moderate at best, with AUCs not exceeding 75%. Furthermore, for the differentiation between VC and Ctrl, the AUCs of both tests showed confidence intervals encompassing 50%, indicating non-efficient discrimination.
Table 3: Discriminant value of MMSE and MDRS-IP tested by ROC analysis (95% confidence interval).
|
|
Cut-off |
Sensitivity |
Specificity |
PLR |
NLR |
AUC |
|
MMSE |
||||||
|
VCDD vs Ctrl |
≤ 28 |
1 (1-1) |
0.85 (0.55-0.98) |
6.50 |
0.00 |
0.96 (0.87-0.99) |
|
VCDD vs VC |
≤ 25 |
0.83 (0.68-0.93) |
0.98 (0.89-0.99) |
40.63 |
0.17 |
0.97 (0.91-0.99)a |
|
VCDM vs Ctrl |
≤ 28 |
0.68 (0.53-0.81) |
0.85 (0.55-0.98) |
4.43 |
0.38 |
0.75 (0.62-0.85) |
|
VCDM vs VC |
≤ 28 |
0.68 (0.53-0.81) |
0.65 (0.50-0.78) |
1.96 |
0.49 |
0.71 (0.61-0.80) |
|
VC vs Ctrl |
≤ 28 |
0.35 (0.22-0.50) |
0.85 (0.55-0.98) |
2.26 |
0.77 |
0.54 (0.41-0.67) |
|
VCDD vs VCDM |
≤ 26 |
0.90 (0.77-0.97) |
0.72 (0.57-0.84) |
3.26 |
0.13 |
0.89 (0.81-0.95)b |
|
MDRS-IP |
||||||
|
VCDD vs Ctrl |
≤ 28 |
0.80 (0.64-0.91) |
0.83 (0.52-0.97) |
4.77 |
0.25 |
0.90 (0.78-0.96) |
|
VCDD vs VC |
≤ 26 |
0.77 (0.61-0.89) |
0.78 (0.64-0.89) |
3.54 |
0.29 |
0.84 (0.74-0.91) |
|
VCDM vs Ctrl |
≤ 31 |
0.58 (0.42-0.73) |
0.75 (0.43-0.94) |
2.33 |
0.56 |
0.69 (0.55-0.80) |
|
VCDM vs VC |
≤ 35 |
0.84 (0.69-0.93) |
0.41 (0.27-0.57) |
1.43 |
0.39 |
0.65 (0.54-0.75) |
|
VC vs Ctrl |
< 35 |
0.41 (0.27-0.57) |
0.75 (0.43-0.94) |
1.65 |
0.78 |
0.48 (0.35-0.61) |
|
VCDD vs VCDM |
≤ 23 |
0.62 (0.45-0.77) |
0.77 (0.61-0.88) |
2.65 |
0.50 |
0.73 (0.63-0.83) |
|
PLR=positive likelihood ratio, NLR=negative likelihood ratio, AUC=area under the ROC curve. a P=0.002 vs MDRS-IP. b P=0.004 vs MDRS-IP |
||||||
DISCUSSION
The results of the present study indicate that both MMSE and MDRS-IP perform better at the dementia level of VCD, being effective in the discrimination of vascular dementia from normal subjects or vascular controls (although, for the latter discrimination, MMSE may perform slightly better). However, in patients with vascular mild cognitive impairment, MMSE and MDRS-IP have statistically significant, but clinically moderate ability to differentiate from normal subjects or vascular controls, i.e. to identify cognitive dysfunction in early stages. Finally, both tests fail in the discrimination between vascular and normal controls, indicating that they are not sensitive enough to identify patients with cerebrovascular disease and subclinical cognitive dysfunction.
Previous studies have shown that MDRS-IP, may be useful in the discrimination between controls and patients with post-lacunar stroke dementia [9] or cognitive dysfunction after stroke associated with small vessel disease) [13]. Additionally, other short frontal tests may be useful in vascular dementia, [14,15] and equally effective with MDRS-IP in the discrimination between stroke associated with small vessel disease and controls [16]. Moderate [17] or low [18] diagnostic value in executive dysfunction after small subcortical infarcts has been described by some authors. However, incident lacunes in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) do affect the score of MDRS-IP [19]. After acute infarcts, the score of MDRS-IP is affected negatively by the load of ischemic lesions in basal ganglia and corona radiate [20] and by the degree of (non-Alzheimer’s) frontal atrophy [21]. Discrepancies between studies may be due to differences in the populations studied (post-stroke, post-lacunar infarction, subcortical small vessel disease), the degree of cognitive impairment (vascular dementia, various levels of cognitive dysfunction) and the criteria used for the diagnosis and classification of vascular cognitive disorder. In the present study, included were patients with all subtypes of vascular cognitive decline, in an effort to achieve a population as representative as possible of the true patient population. Furthermore, in an effort to explore the entire cognitive continuum of cerebrovascular disease, we subdivided patients according to the degree of cognitive decline, in vascular dementia (VCDD) and a pre-dementia symptomatic group (vascular mild cognitive impairment, VCDM) and, additionally, we used a preclinical vascular control group (VC) in which cerebrovascular disease has not resulted yet in clinically evident cognitive dysfunction.
CONCLUSION
In conclusion, both MMSE and MDRS-IP, are comparably useful in vascular cognitive disorder of dementia severity. In less severely affected patients their diagnostic value decreases and in clinically unaffected patients they are not sensitive tools. Other neuropsychological tools, alone or in combination, may be needed in order to achieve early recognition of cognitive dysfunction in non-dementia patients.
Conflict of Interest: The authors declare no conflict of interest.
REFERENCES
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American heart association/American stroke association. Stroke 42(9): 2672-713.
- Sachdev P, Kalaria R, O’Brien J, Skoog I, Alladi S, Black SE, et al. (2014) Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 28(3): 206-218.
- Skrobot OA, Black SE, Chen C, DeCarli C, Erkinjuntti T, Ford GA, et al. (2018) Progress toward standardized diagnosis of vascular cognitive impairment: Guidelines from the vascular impairment of cognition classification consensus study. Alzheimers Dement 14(3): 280-292.
- Zhou A, Jia J (2009) A screen for cognitive assessments for patients with vascular cognitive impairment no dementia. In J Geriatr Psychiatry 24(12): 1352-1357.
- Erkinjuntti T (2002) Subcortical vascular dementia. Cerebrovasc Dis 13(Suppl 2): 58-60.
- Wallin A, Román GC, Esiri M, Kettunen P, Svensson J, Paraskevas GP, et al. (2018) Update on vascular cognitive impairment associated with subcortical small-vessel disease. J Alzheimers Dis 62(3): 1417-1441.
- Mattis S (1976) Mental status examination for organic mental syndrome in the elderly patients. In: Bellack L, Karasu TB, eds. Geriatric Psychiatry: A handbook for psychiatrists and primary care physicians. Grune & Stratton, New York, USA 77-121.
- Kramer JH, Reed BR, Mungas D, Weiner MW, Chui HC (2002) Executive dysfunction in subcortical ischaemic vascular disease. J Neurol Neurosurg Psychiatry 72(2): 217-220.
- Tang WK, Mok V, Chan SS, Chiu HFK, Wong KS, Kwok TC, et al. (2005) Screening of dementia in stroke patients with lacunar infarcts: Comparison of the Mattis dementia rating scale and the mini-mental state examination. J Geriatr Psychiatry Neurol 18(1): 3-7.
- Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, et al. (1992) Atrophy of the medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal aging: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 55(10): 967-972.
- Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. (2018) NIA-AA Research framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement 14(4): 535-562.
- Folstein M, Folstein S, McHugh PR (1975) “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3): 189-198.
- Mok VC, Wong A, Lam WW, Fan YH, Tang WK, Kwok T, et al. (2004) Cognitive impairment and functional outcome after stroke associated with small vessel disease. J Neurol Neurosurg Psychiatry 75(4): 560-566.
- Oguro H, Yamaguchi S, Abe S, Ishida Y, Bokura H, Kobayashi S (2006) Differentiating Alzheimer’s disease from subcortical vascular dementia with the FAB test. J Neurol 253(11): 1490-1494.
- Kugo A, Terada S, Ata T, Ido Y, Kado Y, Ishihara T, et al. (2007) Japanese version of the frontal assessment battery for dementia. Psychiatry Res 153(1): 69-75.
- Wong A, Mok VC, Tang WK, Lam WW, Wong KS (2007) Comparing Mattis dementia rating scale-initiation/perseveration subset and frontal assessment battery in stroke associated with small vessel disease. J Clin Exp Neuropsychol 29(2): 160-9.
- Mok VC, Wong A, Yim P, Fu M, Lam WW, Hui AC, et al. (2004) The validity and reliability of Chinese frontal assessment battery in evaluating executive dysfunction among Chinese patients with small subcortical infarct. Alzheimer Dis Assoc Disord 18(2): 68-74.
- Wong A, Mok VC, Yim P, Fu M, Lam WW, Yau C, et al. (2004) The executive clock drawing task (CLOX) is a poor screening test for executive dysfunction in Chinese elderly patients with subcortical ischemic vascular disease. J Clin Neurosci 11(5): 493-497.
- Ling Y, De Guio F, Duering M, Jouvent E, Hervé D, Godin O, et al. (2017) Predictors and clinical impact of incident lacunes in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke 48(2): 283-289.
- Hua P, Pan XP, Hu R, Mo XE, Shang XY, Yang SR (2014) Factors related to executive dysfunction after acute infarct. PLoS One 9(9): e108574.
- Mok V, Wong KK, Xiong Y, Wong A, Schmidt R, Chu W,et al. (2011) Cortical and frontal atrophy are associated with cognitive impairment in age-related confluent white-matter lesion. J Neurol Neurosurg Psychiatry 82(1): 52-57.
Copyright: Paraskevas GP, et al. ©2019. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Paraskevas GP (2019). The diagnostic value of Mattis Dementia Rating Scale-Initiation Perseveration Subscale in Vascular Cognitive Disorders. Neuro Research 1(1): 2.
 Abstract
Abstract  PDF
PDF