Past Issues
The Role of Intravenous Immunoglobulins in Treatment of Viral Encephalitis in Children
Sameh A. Abd EL-Naby#, Nagwan Y. saleh, Nahla M. Said, Esraa K. EL-Baz*
Pediatrics Department, Faculty of Medicine, Menoufia University, Egypt
Corresponding author
*Esraa Kamal EL-Baz, Ashmoun City, Menoufia Governorate, Egypt, Tel: 010-095-700-71; Fax: 048-342-0726; Email: [email protected]. #Sameh Abd Allah Abd EL-Naby, Department of pediatrics, Menoufia University, Shebin El-kom, Egypt, Tel: 010-680-679-64; E-mail: [email protected]. Received : August 25, 2020 Published : September 22, 2020
ABSTRACT
Objectives: To determine the efficacy and safety of intravenous immunoglobulin (IVIG) as add on therapy in treatment of viral encephalitis in children.
Background: Encephalitis is a syndrome of neurological dysfunction that results from inflammation of brain parenchyma, caused by an infection or an exaggerated host immune response, or both. Attenuation of brain inflammation through modulation of the immune response could improve patient outcomes. Biological agents such as immunoglobulin have anti-inflammatory and immunomodulatory properties therefore may be useful as add on therapy in treatment of children with encephalitis.
Material and Methods: 60 children with encephalitis were enrolled through a randomized controlled trial, 30 children received intravenous immunoglobulins (IVIG) as add on standard therapy of encephalitis and 30 children received standard therapy only. Duration of hospital admission and post encephalitic sequelae were assessed and followed.
Results: Children with viral encephalitis who received IVIG as add on standard therapy (group1) and who received standard therapy only (group2) showed no significant difference regarding mortality (P0.67). No significant difference regarding post encephalitic sequelae (P0.461).No significant difference regarding duration of hospital admission (P0.093).
Conclusion: The study suggests no clinical benefit of using IVIG as add on therapy in treatment of pediatric viral encephalitis regarding mortality, duration of hospital admission, and post-encephalitic sequelae. Despite these findings, the risk of bias in the included studies and quality of the evidence make it impossible to reach any firm conclusions on the efficacy and safety of IVIG as add on therapy in treatment of pediatric encephalitis. Consideration should be given to a large sample size with statistical power to detect clinically significant differences in theses outcomes and longer term follow up.
KEYWORDS: Encephalitis; Intravenous immunoglobulin; Pediatric
INTRODUCTION
Encephalitis is a syndrome of neurological dysfunction that results from inflammation of the brain parenchyma [1]. The worldwide annual incidence ranges from 3.5 to 7.4 under one year of age. In 2013, the International Encephalitis Consortium (IEC) created simplified consensus diagnostic criteria for a standardized case definition of encephalitis. Altered mental status for over 24 hours without an alternative cause is required as evidence of neurologic dysfunction. In addition, supplemental minor criteria must be present (2 for possible, ≥ 3 for probable or confirmed): fever ≥ 38°C within 72 hours, seizures, new focal neurologic findings, cerebrospinal fluid (CSF) pleocytosis (≥ 5 white blood cells/μL), neuroimaging with brain parenchymal changes or electroencephalogram (EEG) consistent with encephalitis [2]. Encephalitis could result from an infection of the brain (infectious encephalitis) or from autoantibodies that affect the brain (immunemediated encephalitis), or both [3]. Infection has been considered the major cause of encephalitis and more than 100 different causative pathogens have been recognized. Viruses are the most common pathogens known to cause encephalitis. However, a host of other pathogens including bacteria and protozoa have also been implicated [4]. Immune-mediated disorders, such as acute disseminated encephalomyelitis (ADEM) recognized to contribute to a significant proportion of cases where no infective cause is identified [5]. Several well-characterized immunological syndromes that are mediated by antibodies against central nervous system surface proteins such as the N-methyl-D-aspartate receptor and the voltage-gated potassium channel-complex and its associated proteins have been identified in people with encephalitis [6]. And these account for 4 and 7% of overall cases [5]. An exaggerated host immune response has been implicated in the pathogenesis of encephalitis and this has been shown to play a part in the disease pathogenesis [7]. IVIG is being used increasingly in the management of a wide range of neurological conditions and its efficacy has been established in a few of these [8]. It has a half- life of three to four weeks. IN encephalitis attenuation of brain inflammation through modulation of the immune response could improve patient outcomes [2]. Biological agents such as immunoglobulin that have both anti-inflammatory and immunomodulatory properties may therefore be useful as adjunctive therapy for Encephalitis [9]. Acyclovir is widely used in the treatment people with herpes virus infections, particularly herpes simplex and varicella-zoster virus. The role of corticosteroid in the treatment of encephalitis is not yet established. Although not routinely used in herpes simplex virus encephalitis (HSVE), corticosteroids are often used in people with HSVE with marked cerebral edema, brain shift, or raised intracranial pressure. Information from clinical observations [10,11] indicates a substantial benefit in outcomes for people with HSVE treated with adjuvant dexamethasone. Corticosteroids are potent anti?inflammatory agents. Results of a prospective randomized controlled trial (RCT) on the use of adjunctive corticosteroid therapy in HSVE are awaited [12]. In present study we studied the role of IVIG as add on standard therapy of children with viral encephalitis.
PATIENTS AND METHODS
This study was a randomized controlled trial that carried out at Menoufia university Hospital during the period from October 2018 to October 2019on (60) children with viral encephalitis [30 males, 30 females] after ethics committee and ethical practices permission, their mean age was 4.41 ± 4.3. The sixty children with encephalitis were classified into two groups. The first group enrolled (30) children [16 males, 14 females], their mean age was 3.89 ± 2.58 and they received IVIG as add on standard therapy. The second group enrolled (30) children [14 males, 16 females], their mean age was 2.93 ± 1.52 and they received standard therapy only. The diagnosis based on history, clinical examination, diagnostic criteria of encephalitis (possible and confirmed encephalitis) regarding (fever, new onset seizure, altered mental status, cerebrospinal (CSF) leukocyte, new onset of focal finding, new onset lesion in brain imaging, electroencephalogram (EEG finding), routine investigations (complete blood count (CBC), organ functions and electrolyte).
We excluded from this study children with other causes of coma as hepatic encephalopathy, uremic encephalopathy, traumatic brain injury, Children with chronic encephalitis (i.e. where presenting symptoms had lasted longer than four weeks) and Children with known hypersensitivity to IVIG.
All the studied groups were subjected to detailed history taking with special emphasis on development of altered mental status, development and onset of fever, new onset seizure and focal lesion, complete neurological examination (Glasgow coma scale, mental status, cranial nerves examinations, muscle tone, muscle power, reflexes and sensory system affection) and the laboratory work-up including 1 ml of blood was collected in EDTA-containing tubes for complete blood count (CBC) by a Sysmex XN-1000 (Japan ;19723), 2 ml of venous blood was obtained from each subject by venipuncture; and was put into a plain tube and left to clot and then centrifuged for 10 min at 4000 rpm. The serum that was obtained was utilized for estimating liver enzymes (ALT and AST) by kinetic UV advanced strategy IFCC (LTEC kit, England) and serum creatinine by DIAMOND Diagnostics, Germany kits and CSF was collected at time of admission for all cases with comment on CSF pressure, color, density and each sample examined microscopically for WBC count and CSF culture. The DNA was extracted from CSF using Thermo Scientific Gene JET Whole Blood Genomic DNA Purification Mini Kit (K0781) according to the manufacturer’s instructions.
Qualitative data was expressed as number and percent. Non-normally distributed continuous variables were presented as median and range (minimum–maximum). Chi-square test or Fisher exact test were used, as indicated, to assess association between qualitative variables. Mann-Whitney U test was used for comparison between two non-normally distributed continuous variables. A two-tailed-value <0.05 was considered statistically significant. Collected data was analyzed using SPSS version 23 (Statistical Package for Social Science) (Chicago, Inc, Illinose).
RESULTS
In terms of the demographic data, there were no significant difference between patients groups regarding age, sex, residence and consanguinity (Table 1).
|
|
Group I N =30 |
Group II N=30 |
Test of significance |
P - value |
||
|
N |
% |
N |
% |
|||
|
Age (years) Mean ± SD Minimum-Maximum Median |
3.89±2.58 0.3-16 4 |
2.93±1.52 0.1-15 1.5 |
U 1.76 |
0.084 NS |
||
|
Sex Male Female |
16 14 |
53.3 46.7 |
14 16 |
46.7 53.3 |
χ2 0.26 |
0.79 NS |
|
Consanguinity Positive Negative |
4 26 |
13.3 86.7 |
6 24 |
20 80 |
χ2 0.48 |
0.73 NS |
|
Motor development Normal Delayed |
30 0 |
100 0 |
28 2 |
92.9 7.1 |
FXT
2.2 |
0.22 NS |
|
Mental development Normal Delayed |
29 1 |
96.7 3.3 |
28 2 |
92.9 7.1 |
FXT
0.42 |
0.47 NS |
Table 1: Demographic and developmental of the two studied groups. χ2 = Chi-Square test; FXT = Fishers exact test; U = Mann-Whitney test, SD = standard deviation, S = significant, NS = non-significant.
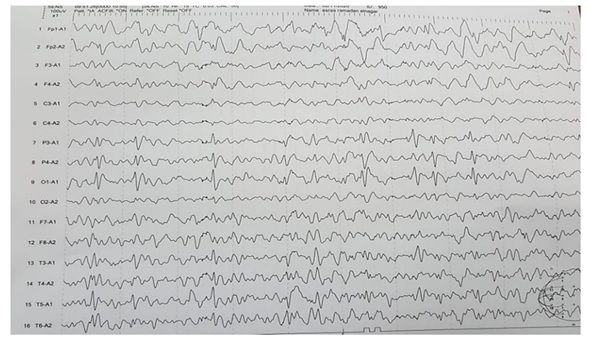
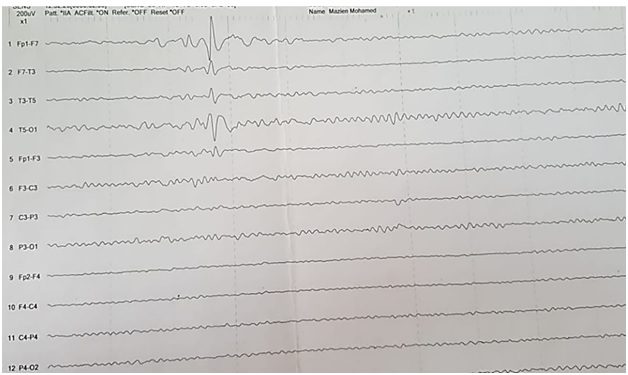
Neurological examination showed no significant difference between patient groups in Glasgow coma scale P0.29 (median score 13 ranged 3 to 15 in group 1 and median score 13 ranged 6 to 15 in group 2), cranial nerves, tone, reflexes and sensory system examination. Patients were diagnosed as possible and confirmed encephalitis according to diagnostic criteria of encephalitis and showed no significant difference regarding new onset seizure P0.27, focal neurological finding P0.38, new onset lesion in brain imaging P0.43 and CSF leucocyte P0.09, however there was a significant difference in altered mental status P0.01, grade of fever P0.001 and EEG finding P0.029 (Table2) and we showed normal EEG finding in (Figure 1) and showed periodic lateralized epileptiform discharges (PLEDs) in (Figure 2).
|
|
Group I N=30 |
Group II N=30 |
||
|
N |
% |
N |
% |
|
|
Fever Mean ± SD Minimum-Maximum Median |
38.35±0.55 37-39 38.5 |
38.86±0.47 38-40 38.5 |
||
|
New onset of seizure Yes No |
21 9 |
70 30 |
24 6 |
80 20 |
|
Altered mental status Decreaseconscious level Drowsy Personal changes |
20 10 0 |
66.7 33.3 0 |
25 1 4 |
83.3 3.3 13.3 |
|
cerebrospinal fluid leukocyte ≤5 ?5 |
14 16 |
46.7 53.3 |
8 22 |
26.7 73.3 |
|
New onset of focal finding Yes No |
10 20 |
33.3 66.7 |
6 24 |
20 80 |
|
Electroencephalogram finding Not done Normal Diffused slowing Left temporal spikes Local focal lesion Frontal slowing Generalized epileptogenic activity |
21 1 5 1 1 1 0 |
(70%) (3.3%) (16.7%) (3.3%) (3.3%) (3.3%) (0%) |
26 3 0 0 0 0 1 |
(86.7%) (10%) (0%) (0%) (0%) (0%) (3.3%) |
|
New onset lesion in brain imaging: Yes No |
15 15 |
50 50 |
11 19 |
36.7 63.3 |
|
Diagnosis Conformed encephalitis Possible encephalitis |
24 6 |
80 20 |
22 8 |
73.3 26.7 |
Table 2: Diagnostic criteria of encephalitis of the two groups.
Figure 1: A case with generalized epileptogenic activity.
Figure 2: A case of left temporal epileptogenic activity.
Laboratory investigations showed no statistical difference between the two groups in white blood count, alanine transferase, aspartate transferase, sodium and potassium levels, hemoglobin levels P0.68, platelet count P0.52, Creatinine P0.64 and calcium levels P0.084 (Table 3).
|
|
Group I N=30 |
Group II N=30 |
Test of significance |
P - value |
|
Hemoglobin Mean ± SD Minimum.-Maximum Median |
11.25±1.2 8.1-13 11.5 |
11.12±1.3 8-14.3 11 |
t 0.4 |
0. 68 NS |
|
White blood count Mean ± SD Minimum.-Maximum Median |
17.43±5.24 9.4-30.1 17.1 |
18.72±10.9 5.2-66 17.5 |
U
0.36
|
0.71 NS |
|
Platelets Mean ± SD Minimum.-Maximum Median |
375.6±121.4 174-768 348.5 |
356.2±111.5 166-567 332.6 |
U
.64
|
0.52 NS |
|
AST Mean ± SD Minimum.-Maximum Median |
31.66±20.7 12-100 23 |
40.95±35.8 11-200 31.7 |
U
1.19 |
0.23 NS |
|
ALT Mean ± SD Minimum.-Maximum Median |
50.23±28.31 13-120 40.5 |
64.01±68.90 13-340 46.4 |
U
0.45 |
0.64 NS |
|
Creatinine Mean ± SD Minimum.-Maximum Median |
0.50±0.15 0.3-0.8 0.50 |
0.56±0.26 0.2-1.1 0.50 |
U 0.45 |
0.64 NS |
Table 3: Routine laboratory investigations of the two groups. t = students t test, U = Mann-Whitney test, SD = standard deviation, S = significant, NS = non-significant.
Duration of hospital admission was calculated and patients were followed up for assessment of disability and post encephalitic sequelae. There was no significant difference regarding outcomes (mortality) P 0.67, significant disability among survivals P = 0.08 and duration of hospital admission (Mean ± SD = 21.86 ± 14.49 in group1 and (Mean ± SD = 29.40 ± 21.49) in group 2 P0.093 (Table 4).
|
|
Group I N=30 |
Group II N=30 |
Test of significance |
P value |
||
|
N |
% |
N |
% |
|||
|
Outcome Survive Died |
28 2 |
93.3 6.7 |
26 4 |
86.7 13.3 |
FXT
0.74 |
0.67 NS |
|
Post-encephalitic sequelae among survival Yes No |
10 18 |
35.7 64.3 |
15 11 |
57.7 42.3 |
χ2
2.61 |
0.08 NS |
|
Duration of hospital admission (days) Mean ± SD Minimum-Maximum Median |
21.86±14.49 5-61 19 |
29.40±21.49 6-90 26 |
U 1.68 |
0.093 NS |
||
Table 4: Outcomes, post-encephalitic sequelae and duration of hospital admission of the two groups. U = Mann-Whitney test, χ2 Chi-Square test, FXT = Fishers exact test, S = significant, NS = non-significant, SD = standard deviation.
DISCUSSION
Encephalitis is a syndrome of neurological dysfunction that results from inflammation of brain parenchyma [1]. Biological agents such as immunoglobulin that have both anti-inflammatory and immunomodulatory properties may therefore be useful in Encephalitis [9].
Regarding the demographic data there were no significant difference between patients groups as regarding age, sex, residence and consanguinity.
In our study the age of the enrolled children ranged between 1month and 16 years old with Mean ± SD of 4.41 ± 4.3 who subdivided into two groups group 1 who received IVIG added on standard therapy in which the age ranged between 3 months and 16 years old with Mean ± SD of 3.89 ± 2.58 and the other group who received standard therapy only (group2) the age of the enrolled children ranged between 1 month old and 15 years old with Mean ± SD of 2.93 ± 1.52, while in Rayamajhi A, et al. [13] the age of enrolled children ranged between 1 year and 14 years. Also in Wu et al. [14] the age of enrolled children ranged between 1 year and 6 years old. Also in Chen DM, et al. [15] the age of enrolled children ranged between 8 months and 12 years old.
Diagnosis of acute encephalitis is not always easily interpreted and CNS symptoms might initially be vague, especially in young children. In our study the Mean ± SD of fever was (38.60 ± 0.57), while in Fowler A, et al. [16] in study I (80%) of the children with acute encephalitis had fever or a recent history of fever at the time of presentation. Altered mental status might be difficult to assess in a child with high fever, and even more difficult in young children, and repeated evaluations are often necessary. In our study altered mental status was seen in 100% of patients, however was seen in 80% of children Fowler A, et al. [16]. New onset of seizure was seen in 25% of patients and new onset of focal finding was seen in 26.7 of patients in our study, while in Fowler A, et al. [16], Wang IJ, et al. [17], Iff T, et al. [18] and Doja A, et al. [19] focal neurological findings and seizures were seen in approximately 40% of the children. CSF leukocyte was ≤5 in 36.7% and ?5 in 63.3% of patients in our study, however in Fowler A, et al. [16] pleocytosis was seen in 55%. EEG showed pathological changes in 69% of cases in whom EEG were performed in our study, while in Fowler A, et al. [16] EEG examinations showed a picture compatible with encephalitis in 90% of children in whom a registration was performed and also pathological changes on EEG examinations in Galanakis E, et al. [20], Wang IJ, et al. [17], Kolski H, et al. [21], and Doja A, et al. [19] have been found in 40-80% of children. New onset brain lesion was found in 43.3% (all of them picture of encephalitis except two cases showed a picture of ADEM of cases in our study, however Fowler A, et al. [16] showed abnormalities compatible with encephalitis in 30% of the children who underwent neuroimaging, four children had an MRI picture compatible with ADEM.
The duration of hospital admission in our study in group 1 ranged between 5 and 61 days with Mean ± SD of 21.86 ± 14.49 and in group 2 ranged between 6 and 90 days with Mean ± SD of 29.40 ± 21.49, while in the study done by Wu H, et al. [14] duration in group 1 was with mean ± SD 7.53 ± 0.83 & group 2 was with mean ± SD of 13.43 ± 1.21. However, in Chen DM, et al. [15] the mean duration was 10.2 ± 3.2 in group 1 and 13.1 ± 4.2 in the other group.
In our study there were 2 children died and 28 survived in group 1 & 4children died and 26 survived in (group2), while in Rayamajhi A, et al. [13] there was 1child died and 10 survived in group 1 & 2 children died and nine children survived (group 2).
With follow up of survivors in our study there were 18 children in group 1 and 11 children in group 2 completely normal. One child developed dysarthria which improved one month after discharge in group 1, however in group 2 there was 2 children developed dysarthria which still does not improve. One child in group1and two children group 2 developed epilepsy. One child in group 1 and two children in group 2 developed spasticity and affection of mentality. One child in group one and one child in group 2 developed affection of mentality. On the other hand, one child in group 1 developed mood liability which improved six months after admission. One child in group1 developed residual ptosis and dysarthria which gradually improved. One child in group 1 developed right eye glaucoma. Two children in group 1 developed inability to walk. One child in group 1 developed Spasticity due to arrest before coming to hospital. One child in group 2 developed pale optic disc. One child in group 2 developed spasticity and affection of mentality & hydrocephalic changes.
One child in group 2 developed slight weakness in right hand. Three children in group 2 developed Spasticity. While in Rayamajhi A, et al. [13] there were eight children in group 1 and 8 children in group 2 developed significant disabilities at discharge. Through the follow up there were three children in group1 developed significant disability at 3–6 months and four children in group 2 developed significant disabilities at 3–6 months. Also in Rayamajhi A, et al. [13] there was one child in group 1 and one child in group 2 developed more than one serious adverse effects. In addition to this there was one child in group 1 and one child in group 2 developed melena. Also there was one child in group1 and one child in group2 developed hypotension.
CONCLUSION
Our study suggests a few beneficial effects of IVIG in reducing post encephalitic sequelae and duration of hospital admission. However due to limitation of this study, we cannot draw any firm and definite conclusion about safety and effectiveness of use of IVIG in treatment of viral encephalitis.
RECOMMENDATION
We recommend that well-designed randomized controlled trials are needed to investigate the role of IVIG in all forms of encephalitis and to provide evidence to inform clinical practice on the management of children with encephalitis. Internationally agreed core outcome measures for clinical trials in childhood encephalitis are still needed. For future trials, Long-term follow-up (beyond the hospitalization period) needs to be assessed. Consideration should be given to a large sample size with statistical power to detect clinically significant differences in these outcomes.
CONFLICT OF INTEREST
There is no conflict of interest.
FUND
No fund was received.
REFERENCES
- Thompson C, Kneen R, Riordan A, Kelly D, Pollard AJ. (2012). Encephalitis in children. Archives of Disease in Childhood. 97:150?61.
- Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, et al. (2013). Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clinical Infection Diseases. 57(8):1114–28.
- Zuliani L, Graus F, Giometto B, Bien C, Vincent A. (2012). Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. Journal of Neurology, Neurosurgery, and Psychiatry. 83:638?45.
- Iro MA, Sadarangani M, Goldacre R, Nickless A, Pollard AJ, et al. (2017). 30?year trends in admission rates for encephalitis in children in England and effect of improved diagnostics and measles?mumps?rubella vaccination: a population?based observational study. Lancet Infectious Diseases. 17(4):422?30.
- Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, et al. (2010). Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population?based prospective study. Lancet Infectious Diseases. 10(12):835?44.
- Hacohen Y, Deiva K, Pettingill P, Waters P, Siddiqui A, et al. (2014). N?methyl?D?aspartate receptor antibodies in post?herpes simplex virus encephalitis neurological relapse. Movement Disorders. 29:90?6.
- Ramakrishna C, Openshaw H, Cantin EM. (2013). The case for immunomodulatory approaches in treating HSV encephalitis. Future Virology. 8(3):259?72.
- Hughes RA, Dalakas MC, Cornblath DR, Latov N, Weksler ME, et al. (2009). Clinical applications of intravenous immunoglobulins in neurology. Clinical and Experimental Immunology. 158(Suppl 1):34?42.
- Rozenberg F. (2013). Acute viral encephalitis. Handbook of Clinical Neurology/Edited by P.J. Vinken and G.W. Bruyn. 112:1171-81.
- Ramos-Estebanez C, Lizarrage KJ, Merenda A. (2014). Asystemic review on the role of adjunctive corticosteroids in herpes simplex virus encephalitis: is timing critical for safety and efficacy? Antiviral Therapy. 19:133-9.
- Lizarraga KJ, Alexandre LC, Ramos-Estebanez C, Merenda A. (2013). Are steroids a beneficial adjunctive therapy in the immunosuppressed patient with herpes simplex virus encephalitis?. Case reports in neurology. 5:52-5.
- Martinez-Torres F, Menon S, Pritsch M, Victor N, Jenetzky E, et al. (2008). Protocol for German trial of Acyclovir and Corticosteroids in Herpes-simplex-virus-Encephalitis (GACHE): a multicenter, multinational, randomized, double-blind, placebo-controlled German, Austrian and Dutch trial ]ISRCTN45122933[. BMC Neurology. 08;8:40.
- Rayamajhi A, Nightingale S, Bhatta NK, Singh R, Ledger E, et al. (2015). A preliminary randomized double blind placebo-controlled trial of intravenous immunoglobulin for Japanese encephalitis in Nepal. Plos One. 10(4):e0122608.
- Wu H, Wan Z, Liu L, Qiu L, Xiuling D. (2014). Comparison of the clinical efficacy of immunoglobulins and interferons in the treatment of hand foot and mouth disease complicated with viral encephalitis. Chinese Journal of Primary Medicine and pharmacy. 21:3127-8.
- Chen D-M, Li S-N. (2006). The clinical observation of high dose immunoglobulin for children viral encephalitis. Chinese Journal of Primary medicine and pharmacy. 13(5):816-7.
- Fowler A, Stodberg T, Eriksson M, Wickstrom R. (2008). Childhood encephalitis in Sweden: etiology, clinical presentation and outcome. Eur J Paediatr Neurol. 12(6):484–490.
- Wang IJ, Lee PI, Huang LM, Chen CJ, Chen CL, et al. (2007). The correlation between neurological evaluations and neurological outcome in acute encephalitis: a hospital-based study. Eur J Paediatr Neurol. 11(2):63-9.
- Iff T, Donati F, Vassella F, Schaad UB, Bianchetti MG. (1998). Acute encephalitis in Swiss children: aetiology and outcome. Eur J Paediatr Neurol. 2(5):233-7.
- Doja A, Bitnum A, Jones ELF, Richardson S, Tellier R, et al. (2006). Pediatric Epstein-Barr virus-associated encephalitis: 10- year review. J Child Neurol. 21(5):384-91.
- Galanakis E, Tzoufi M, Katragkou A, Nakou I, Roilides E. (2009). A prospective multicenter study of childhood encephalitis in Greece. Pediatr Infect Dis J. 28(8):740-2.
- Kolski H, Ford-Jones EL, Richardson S, Petric M, Nelson S, et al. (1998). Etiology of acute childhood encephalitis at The Hospital for Sick Children, Toronto, 1994-1995. Clin Infect Dis. 26(2):398-409.
 Abstract
Abstract  PDF
PDF